About
The Pitt-TraCe is one of four Centers funded by the National Center for Advancing Translational Sciences (NCATS) to qualify MPS platforms as drug development tools with the FDA. Our overall goal is to qualify our patient-derived structured, biomimetic liver acinus microphysiological system (LAMPS) and vascularized LAMPS (vLAMPS) platforms as powerful drug development tools (DDTs) for four contexts of use (CoUs) and make them commercially available for drug developers.
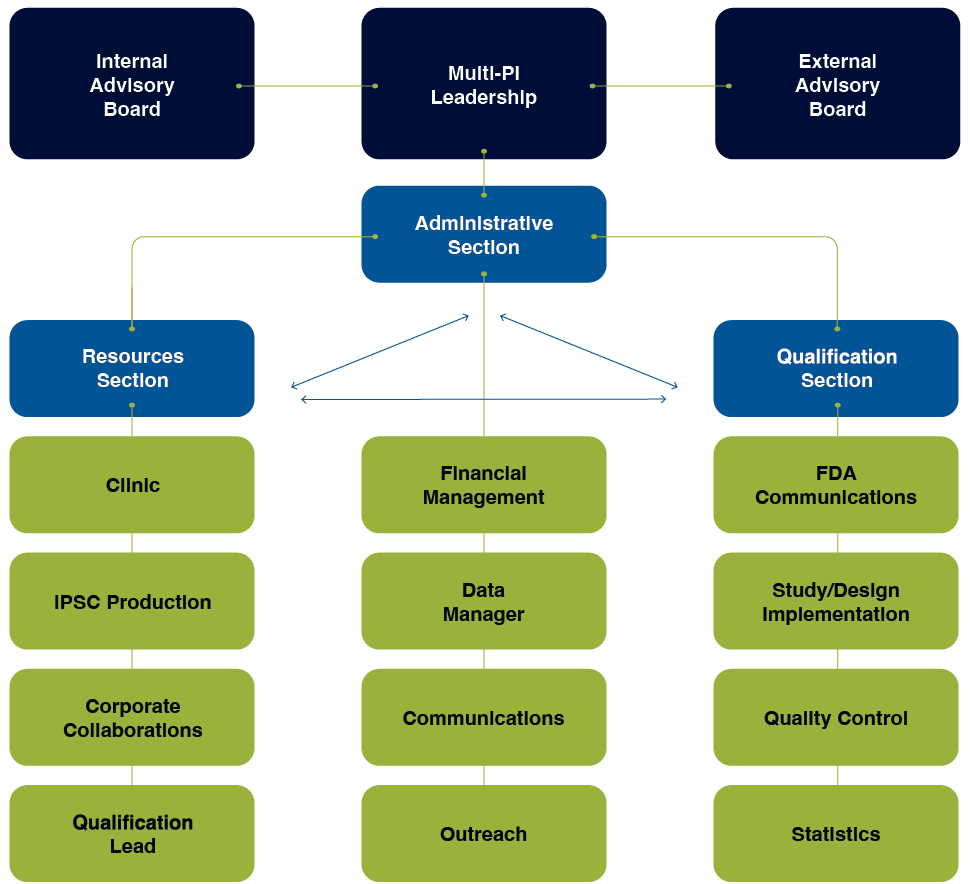
Our organization is comprised of three integrated sections:
Administrative Section
The overall goal of the Administrative Section is to put in place an optimized Center organization and workflow to facilitate qualification of our liver MPS DDTs.
Resources Section
The overall goal of the MPS Resources Section is to establish the sources and quality control (QC) of the Center materials and resources for qualification of DDTs.
Qualification Section
The overall goal of the Qualification Section is to qualify our liver MPS as DDTs to aid in the drug development drugs for metabolic dysfunction-associated steatotic liver disease (MASLD).
We work with the NCATS, FDA, and the Critical Path Institute to qualify our platforms.
Organizational Structure