Pitt Trace
Qualification of patient-derived biomimetic liver MSP as Drug Discovery Tools
Our Mission is to qualify our patient-specific, biomimetic liver Microphysiology Systems (MPS) platforms as powerful drug development tools (DDT) for four distinct contexts of use (CoU) with the ultimate objective of making them accessible for commercial use.
Drug Development Tools
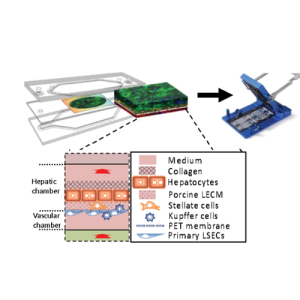
Liver Acinus Microphysiology systems
(LAMPS)
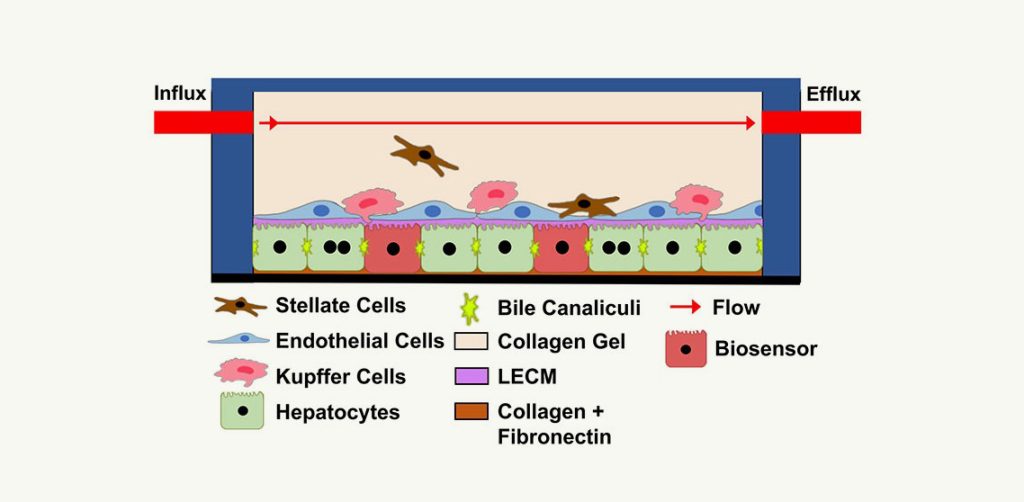
DDT for quantifying hepatic clearance and identification of major metabolites of drug candidates in patients with MASLD.
Context of Use:
The LAMPS as a DDT for establishing CLH of drug candidates in patients with MASLD to provide supporting data to aid in the determination of drug candidate dosing in clinical trials.
DDT for identifying liver toxicity of drug candidates in patients with MASLD.
Context of Use:
The LAMPS as a DDT for establishing the hepatic toxicity of drug candidates in patients with MASLD to provide supporting data to aid in the determination of drug candidate dosing in clinical trials.
Vascularized Liver Acinus Microphysiology systems
(vLAMPS)
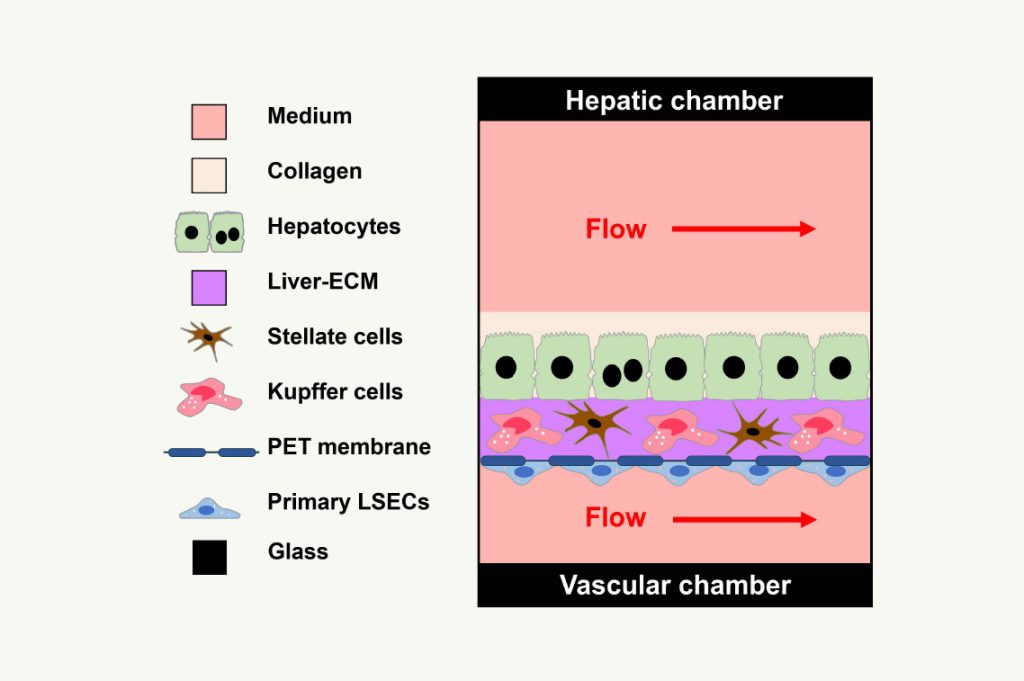
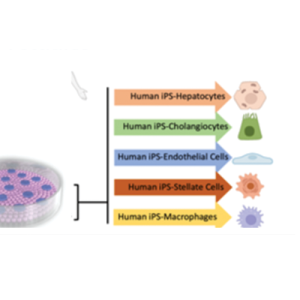
DDT for drug testing using iPSC-derived liver cells from normal subjects maintained as normal (control) or driven to late-stage MASLD (stage 3 fibrosis or earlier) and testing drugs that have been demonstrated to limit progression of steatosis, inflammation, and/or fibrosis.
Context of Use:
The vLAMPS as a DDT for establishing the efficacy of drug candidates in patients with MASLD to provide supporting data to aid in the determination of drug candidate dosing in clinical trials.
DDT for selection of responsive cohorts for clinical trials by employing iPSC-derived liver cells from patients verified to be at late-stage MASLD (stage 3 fibrosis or earlier) and testing drug candidates that have shown patient-specific responsiveness from clinical trials on steatosis, inflammation, and fibrosis and demonstrate concordance with clinical outcomes.
Context of Use:
The vLAMPS as a DDT to be used to predict the responsiveness of individual patients to drug candidates for their inclusion in clinical trials.
Our Focus
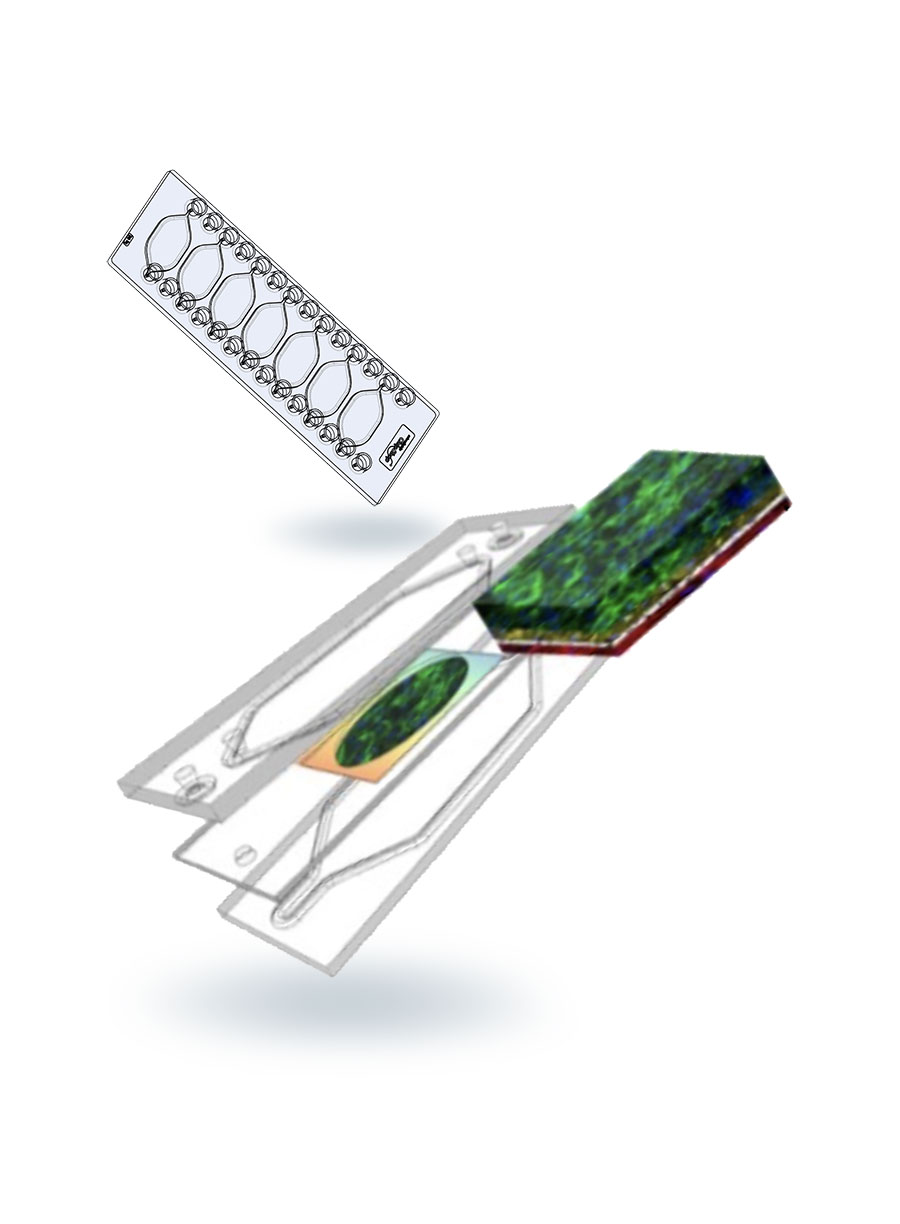
The disease focus of the Center is metabolic dysfunction-associated steatotic liver disease (MASLD), which is a heterogeneous and progressive disease impacting >25% of the world population. The pharmaceutical industry has spent decades applying traditional methods and spending billions of dollars, yet clinical trials have produced only one drug approved by the FDA that is effective in only a minority of patients. We have developed a liver acinus microphysiological system (LAMPS) and a vascularized LAMPS (vLAMPS), and are qualifying them as a drug development tools to aid in the development of drugs for MASLD and other liver diseases.
We are qualifying the LAMPS to:
a) predict candidate hepatic clearance; and b) identify toxicity of drug candidates in patients with MASLD to aid in the assessment of dosing for MASLD clinical trials and provide supporting data showing acceptable toxicity in the human diseased liver for inclusion of the drug candidate MASLD clinical trials.
We are qualifying the vLAMPS to:
a) predict efficacy of drug candidates for resolving MASLD providing data to support advancement to clinical trials and assist in determining dosing; and b) predict the responsiveness of individual patients to drug candidates for their inclusion in clinical trials (clinical trial enrichment).
Our Pipeline
We have established the pipeline and are documenting the protocols for obtaining patient cells to perform qualification 3 and 4 studies.

Our Impact
These DDTs used for these CoUs will address the following FDA regulatory science focus areas:
a) individualized therapies and precision medicine; b) complex innovative trial design; c) technologies to improve predictivity of non-clinical studies; d) technologies to reduce reliance on animal testing; and e) human relevancy of toxicity.
In addition, these DDTs will address the clinical unmet needs for MASLD of:
a) Identifying toxic drugs that should not be given to MASLD patients; b) Identifying the most efficacious drugs to give to MASLD patients; and c) Enrichment of MASLD clinical trials.

